- cross-posted to:
- xkcd@lemmy.world
- cross-posted to:
- xkcd@lemmy.world
I of course understand the joke, but I’ll give someone else the pleasure of explaining it…
Posting this link so I can tap on it.
When posting links to helpful websites rather than answering the question yourself, it’s a good idea to actually read the answer on the helpful website first.
You know, in case there’s a big banner across the top saying “This answer is a useless pile of trash generated by an LLM, please ignore it” (mildly paraphrasing).
The header on explainxkcd is always something funny, because the volunteers are not yet ready to say thst they habe finished the explanation.
The LLM answer was deleted even before I posted the link. As mentioned the header is always a joke, and in this case it’s referencing that, but over the next few days I expect that link to become increasingly useful.
I mean, it wasn’t deleted before you posted the link, because I clicked the link and saw it, but I do see that it’s been updated now.
I mean the actual text that was LLM generated was deleted. The header had been left mentioning it as a joke. Note the discussion section:
“… But I’m gonna go out on a limb and assume this mostly vapid explanation is as good as no explanation, and remove it for now. 108.162.216.132 05:42, 2 January 2025 (UTC)”
It’s all kicking off in the discussion!
Created by CHATGPT FOR SOME REASON - This needs an explanation. Do NOT delete this tag too soon.
But I’m gonna go out on a limb and assume this mostly vapid explanation is as good as no explanation, and remove it for now.
So, all the real nerds are still on holiday?
Finally something I can apply my expensive ChemE degree on :')
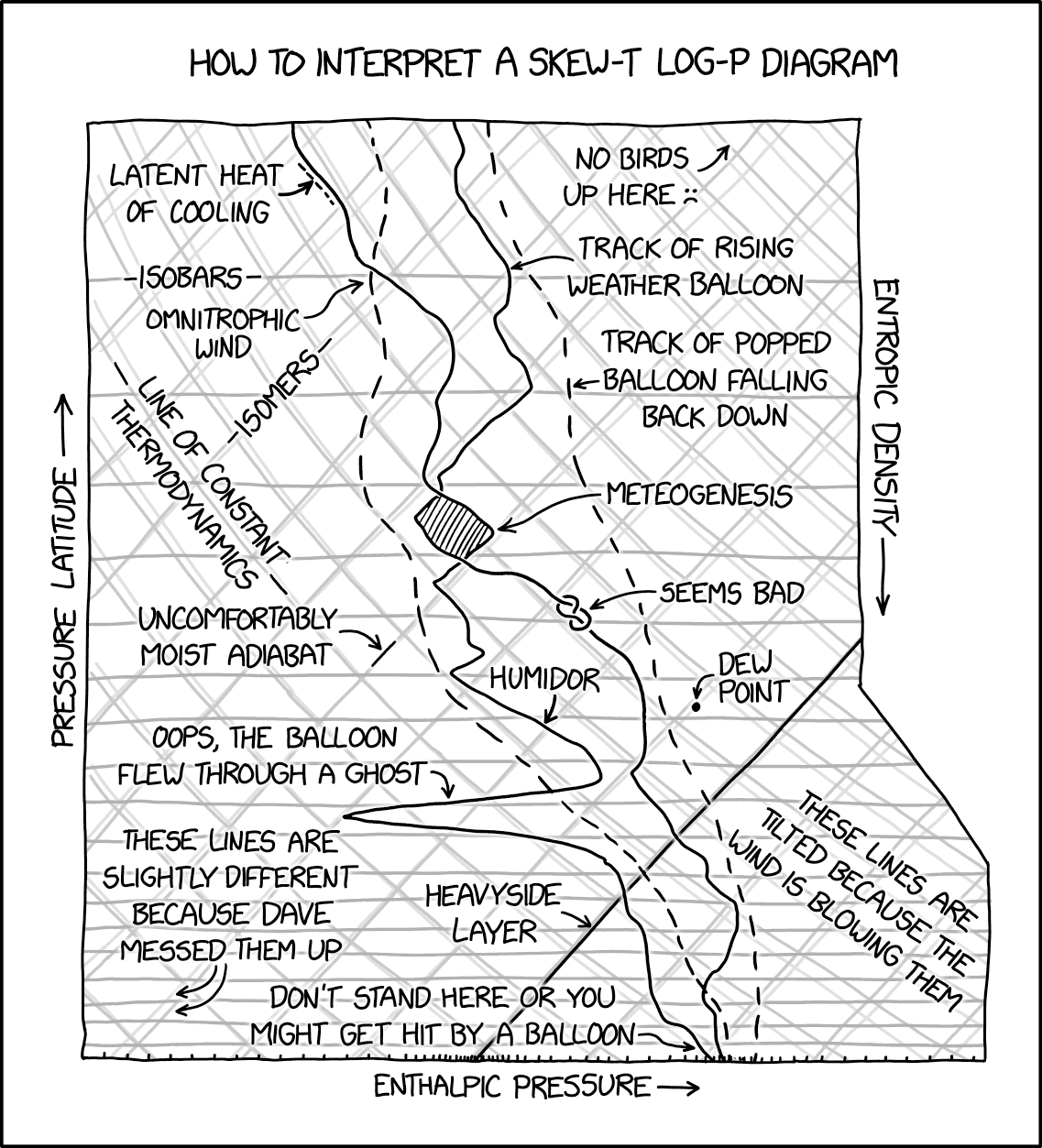
These diagrams were always the worst to use, far too busy and usually printed on cheap paper making it impossible to distinguish one line from the other.
Basically its a diagram is relating the energy required to change the state of something (usually water) and while in theory is amazing and clean in allowing you to determining enthalpy necessary for a state at a constant pressure.
In practice though can get really messy especially with more variables in play, as shown with the temperature decreasing as the balloon altitude increases.