- cross-posted to:
- health@lemmy.world
- news@lemmy.world
- cross-posted to:
- health@lemmy.world
- news@lemmy.world
cross-posted from: https://lemmy.world/post/8344778
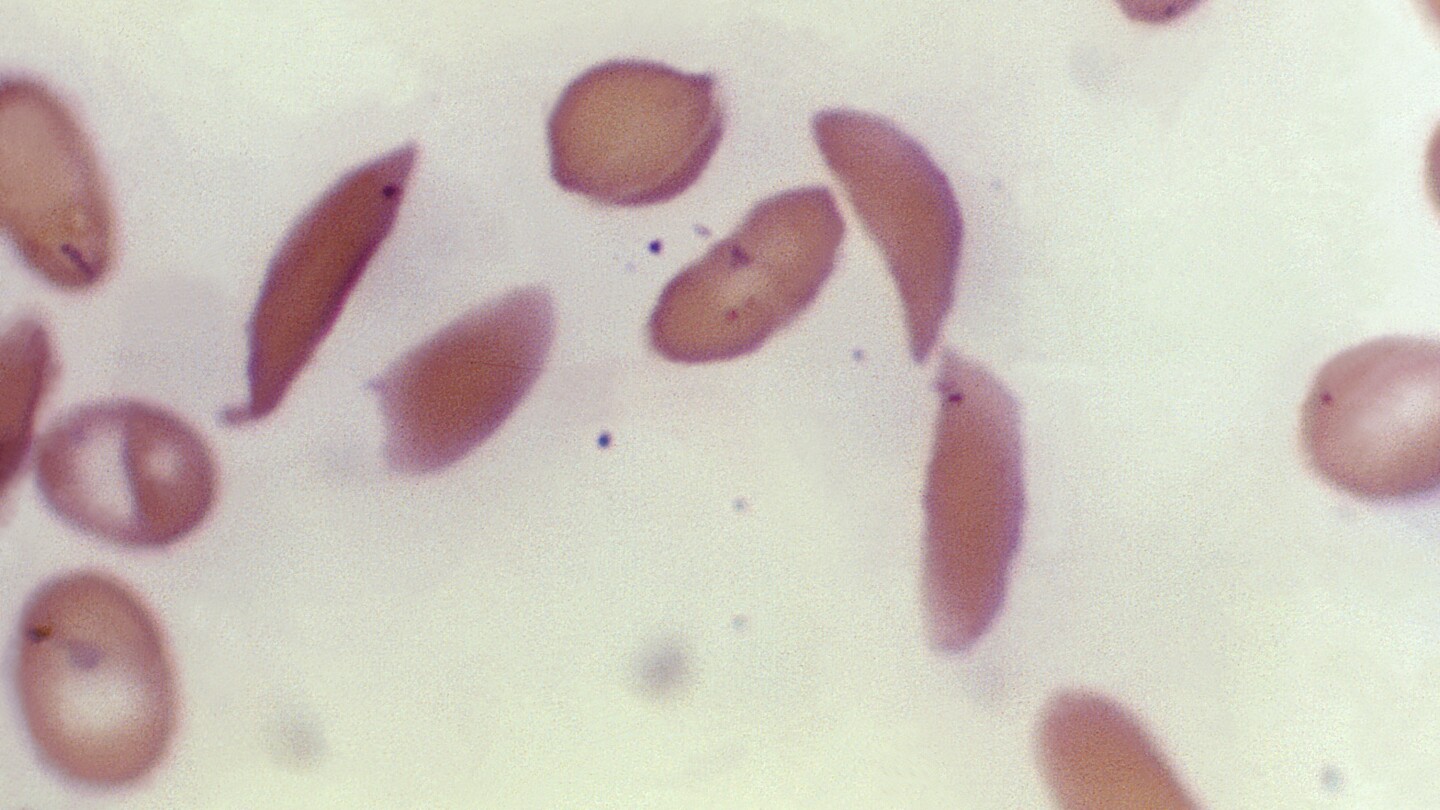
Britain’s medicines regulator has authorized the world’s first gene therapy treatment for sickle cell disease, in a move that could offer relief to thousands of people with the crippling disease in the U.K.
In a statement Thursday, the Medicines and Healthcare Regulatory Agency said it approved Casgevy, the first medicine licensed using the gene editing tool CRISPR, which won its makers a Nobel prize in 2020.
The agency approved the treatment for patients with sickle cell disease and thalassemia who are 12 years old and over. Casgevy is made by Vertex Pharmaceuticals (Europe) Ltd. and CRISPR Therapeutics. To date, bone marrow transplants, extremely arduous procedures that come with very unpleasant side effects, have been the only long-lasting treatment.
“The future of life-changing cures resides in CRISPR based (gene-editing) technology,” said Dr. Helen O’Neill of University College London.


I guess that’s why I’m so excited! If they can do it for sickle cell then it’s possible for MS and EDS! Thanks for confirming that understanding!